With nearly 800 members in the human genome the G protein-coupled receptors (GPCRs) are the biggest family of transmembrane proteins and receptors. All GPCRs consist of seven transmembrane helices, connected by three intercellular and three extracellular loops as well as an extracellular and intracellular domain, including the helix 8. As regulators for blood pressure, the hormone system, and as targets for the treatment of a plethora of diseases including diabetes and adipose, GPCRs are crucial targets in drug discovery. Even though the number of the published GPCR structures is rapidly increasing, the knowledge gap regarding their structures can only be closed via molecular modeling tools such as Rosetta.
In the Meiler Lab we develop pipelines to generate high-quality homology models of these receptors. A modeling protocol based on RosettaGPCR [1] that blends sequence- and structure-based alignment was established. Specific modeling efforts for neuropeptide Y1 receptor [2] and ghrelin bound to GHSR [3] in recent years focused on investigating interaction and binding models.
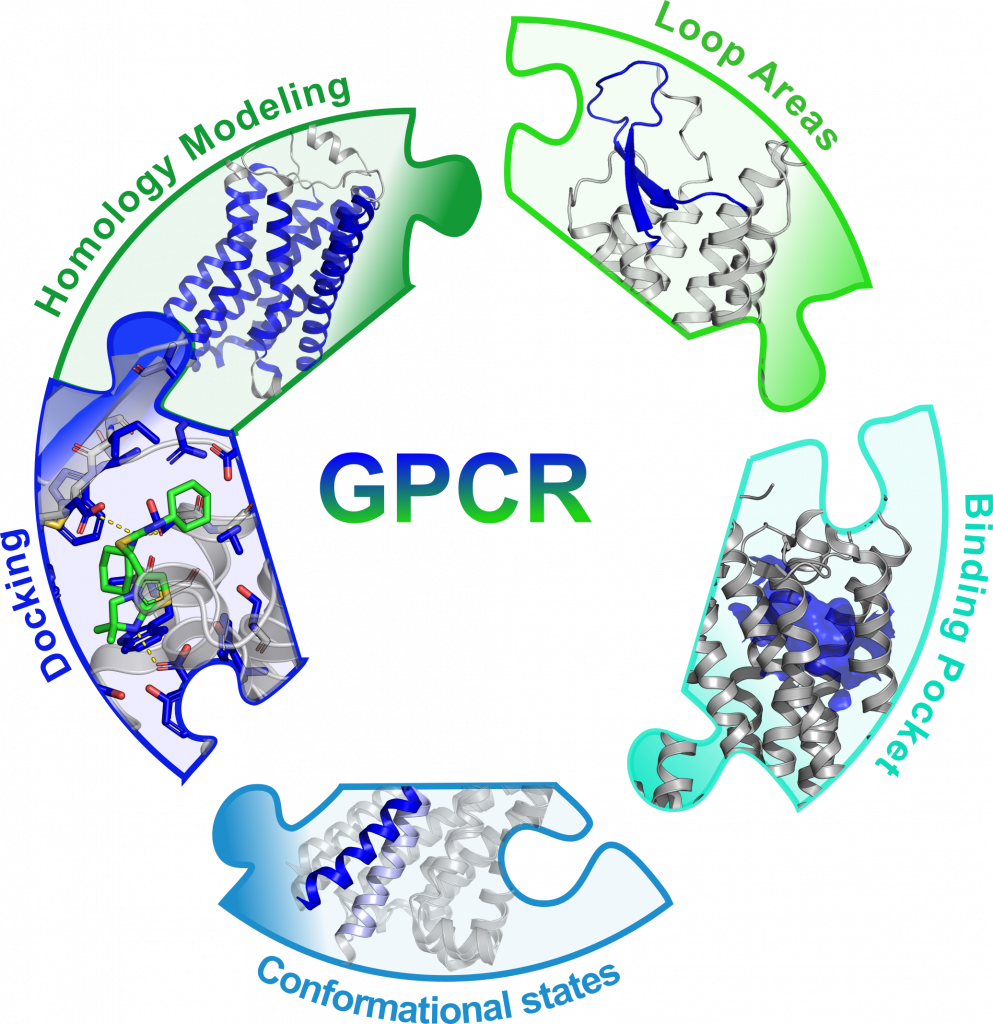
Figure: High quality GPCR modeling pipeline with Rosetta focusing on the most different structural features of the receptors.
REFERENCES:
[1] Bender BJ, Marlow B, Meiler J. Improving homology modeling from low-sequence identity templates in Rosetta: A case study in GPCRs. PLoS Comput Biol. 2020 Oct 28;16(10):e1007597.
[2] Yang Z et al. Structural basis of ligand binding modes at the neuropeptide Y Y1 receptor. Nature. 2018 Apr;556(7702):520-524.
[3] Bender BJ, Vortmeier G et al. Structural Model of Ghrelin Bound to its G Protein-Coupled Receptor. Structure. 2019 Mar 5;27(3):537-544.e4.
